From Itingen to the world. Because health knows no boundaries.
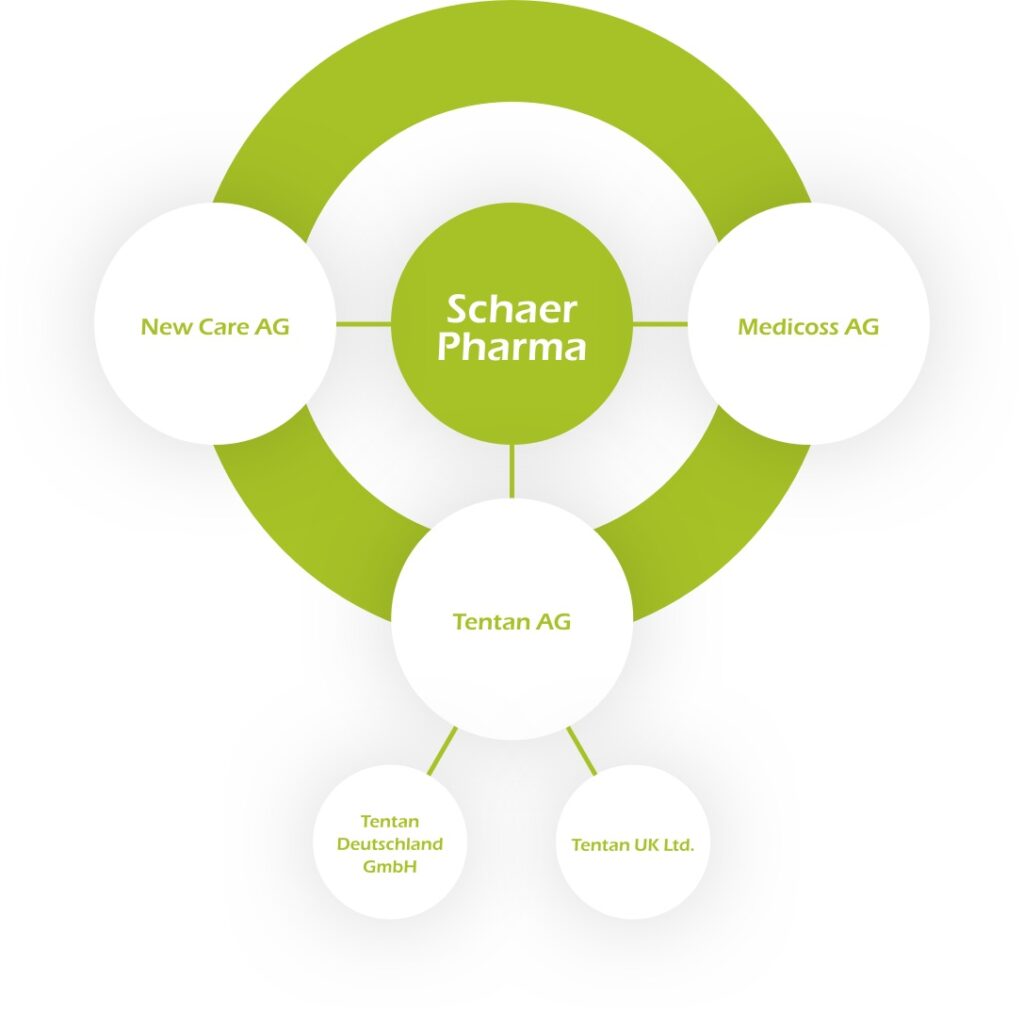
In 1989, Michel Schaer founded Tentan AG, and thus laid the foundation stone for the Schaer Pharma family. Internationally successful with local roots: These two qualities distinguish the corporate group, which has grown steadily over the years. Each expansion step contributes to the overriding goal of keeping channels short and being as close as possible to the market and the people.
Memberships
For consumer welfare and continual development, Schaer Pharma and the companies in the group belong to various industry organisations and associations.
Our certifications
Schaer Pharma markets products from a wide range of product categories that are subject to a variety of regulations. Very high quality standards apply in particular to medicines and medical devices.
To be able to trade in certain product categories, specific certifications must have been obtained. For example, Tentan AG is certified to GMP (Good Manufacturing Practice) standards for the Staad site and to GDP (Good Distribution Practice) standards for the Itingen site. Tentan AG therefore fulfils the qualitative aspects required to be able to trade in various medicines as the marketing authorisation holder and also to package them itself at the Staad site. Furthermore, the two companies New Care AG and Tentan AG at the Itingen site are certified to ISO 13485 and act as manufacturers of a variety of medical devices.











